Abstract
Background
Sickle cell disease (SCD) is an autosomal recessive hemoglobinopathy associated with chronic hemolysis and vaso-occlusive crises (VOCs) resulting in pain, organ damage, and a shortened lifespan. Current treatment options are limited, and many individuals with SCD continue to experience VOCs despite receiving therapy. Although the precise cause of VOCs is not clear, evidence suggests that cell adhesion is involved. Von Willebrand factor (VWF) is a multimeric glycoprotein that mediates the adhesion of platelets to each other and to other cell types, including vascular endothelium and leukocytes. An emerging hypothesis is that VWF contributes to the pathophysiology of VOCs through the formation of hyper-adhesive ultra-large VWF multimers. VWF activity is regulated by the metalloprotease ADAMTS13, which specifically cleaves ultra-large VWF multimers in an extended conformation. Patients with SCD have been shown to have higher levels of VWF multimers and lower levels of ADAMTS13 activity during VOCs. This imbalance could be caused either by the increased generation and release of ultra-large VWF multimers or by the inhibition of ADAMTS13 activity by plasma free hemoglobin or thrombospondin-1. Increasing the plasma concentration of ADAMTS13 using a recombinant ADAMTS13 (rADAMTS13; TAK-755, Takeda Development Center Americas, Inc., Lexington, MA, USA) may be therapeutically beneficial by enhancing cleavage of ultra-large VWF multimers. Here, we report the design and enrollment status of the Recombinant ADAMTS13 In Sickle Cell Disease (RAISE-UP) study (NCT03997760), the first clinical study of a recombinant ADAMTS13 in patients with SCD.
Study Design and Methods
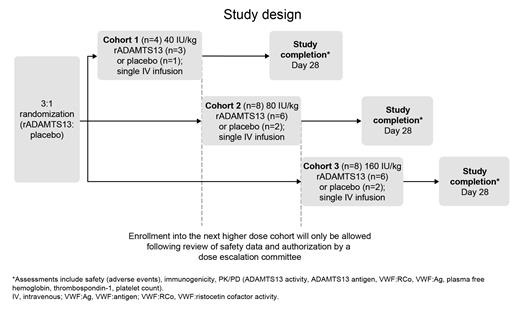
This phase 1, randomized, double-blind, placebo-controlled, multicenter, ascending single dose study will assess the safety (including immunogenicity), tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of rADAMTS13 in patients with SCD. This study is planned to be conducted in 2 parts (part A and part B). Here we present the study design for part A which is being conducted initially and will enroll approximately 20 patients aged between 18 and 65 years with a documented history of SCD (HbSS or HbSβ 0 thalassemia). Concurrent treatment with a stable dose of hydroxyurea is allowed. Exclusion criteria include an acute VOC in the preceding 21 days and a blood transfusion either within the last 30 days or on ≥2 occasions in the last 90 days. Ethics committee approval and patient consent were obtained. Patients will be randomized 3:1 to receive a single intravenous infusion of either rADAMTS13 or placebo in 3 sequential dose cohorts. Patients in cohort 1 (n=4) will receive a 40 IU/kg dose, cohort 2 (n=8) will receive an 80 IU/kg dose, and cohort 3 (n=8) will receive a 160 IU/kg dose (Figure). In cohorts 2 and 3, 6 patients will receive rADAMTS13 and 2 patients will receive placebo. The first 3 patients enrolled in each cohort will be dosed with a separation time of at least 14 days. Enrollment into the next higher dose cohort will only be allowed following review of safety data and authorization by a dose escalation committee. Enrollment will be paused if anaphylaxis, binding or inhibitory antibodies, a life-threatening condition, or death are reported. All patients will complete an end-of-study visit on day 28 following infusion. Primary safety endpoints include adverse events, serious adverse events (SAEs), adverse changes in vital signs and laboratory parameters, and incidence of binding and inhibitory antibodies against rADAMTS13 occurring during the study. A secondary objective is to assess the PK of single-dose rADAMTS13 in each dose cohort, including an assessment of ADAMTS13 antigen and ADAMTS13 activity. Secondary PD objectives are to assess the effect of rADAMTS13 on VWF and platelet count and to study the correlation of plasma free hemoglobin and thrombospondin-1 with rADAMTS13 activity and VWF. Enrollment has been completed for cohort 1. In the review of safety data by the dose escalation committee, no drug-related SAEs were reported and no binding or inhibitory antibodies to ADAMTS13 were observed. On the basis of these findings, cohort 2 has been opened for enrollment.
Patwari: Takeda Development Center Americas, Inc.,: Current Employment. Nguyen: Takeda Development Center Americas, Inc.,: Current Employment. Bhattacharya: Takeda: Current equity holder in publicly-traded company; Takeda Development Center Americas, Inc.: Current Employment. Jain: Takeda Development Center Americas, Inc.,: Current Employment; Takeda: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal